WP_Query Object
(
[query] => Array
(
[post_type] => post
[posts_per_page] => 3
[post_status] => publish
[orderby] => rand
)
[query_vars] => Array
(
[post_type] => post
[posts_per_page] => 3
[post_status] => publish
[orderby] => rand
[error] =>
[m] =>
[p] => 0
[post_parent] =>
[subpost] =>
[subpost_id] =>
[attachment] =>
[attachment_id] => 0
[name] =>
[pagename] =>
[page_id] => 0
[second] =>
[minute] =>
[hour] =>
[day] => 0
[monthnum] => 0
[year] => 0
[w] => 0
[category_name] =>
[tag] =>
[cat] =>
[tag_id] =>
[author] =>
[author_name] =>
[feed] =>
[tb] =>
[paged] => 0
[meta_key] =>
[meta_value] =>
[preview] =>
[s] =>
[sentence] =>
[title] =>
[fields] => all
[menu_order] =>
[embed] =>
[category__in] => Array
(
)
[category__not_in] => Array
(
)
[category__and] => Array
(
)
[post__in] => Array
(
)
[post__not_in] => Array
(
)
[post_name__in] => Array
(
)
[tag__in] => Array
(
)
[tag__not_in] => Array
(
)
[tag__and] => Array
(
)
[tag_slug__in] => Array
(
)
[tag_slug__and] => Array
(
)
[post_parent__in] => Array
(
)
[post_parent__not_in] => Array
(
)
[author__in] => Array
(
)
[author__not_in] => Array
(
)
[search_columns] => Array
(
)
[ignore_sticky_posts] =>
[suppress_filters] =>
[cache_results] => 1
[update_post_term_cache] => 1
[update_menu_item_cache] =>
[lazy_load_term_meta] => 1
[update_post_meta_cache] => 1
[nopaging] =>
[comments_per_page] => 50
[no_found_rows] =>
[order] =>
)
[tax_query] => WP_Tax_Query Object
(
[queries] => Array
(
)
[relation] => AND
[table_aliases:protected] => Array
(
)
[queried_terms] => Array
(
)
[primary_table] => wp_posts
[primary_id_column] => ID
)
[meta_query] => WP_Meta_Query Object
(
[queries] => Array
(
)
[relation] =>
[meta_table] =>
[meta_id_column] =>
[primary_table] =>
[primary_id_column] =>
[table_aliases:protected] => Array
(
)
[clauses:protected] => Array
(
)
[has_or_relation:protected] =>
)
[date_query] =>
[request] => SELECT SQL_CALC_FOUND_ROWS wp_posts.ID
FROM wp_posts
WHERE 1=1 AND wp_posts.post_type = 'post' AND ((wp_posts.post_status = 'publish'))
ORDER BY RAND()
LIMIT 0, 3
[posts] => Array
(
[0] => WP_Post Object
(
[ID] => 4906
[post_author] => 4
[post_date] => 2025-07-17 10:27:09
[post_date_gmt] => 2025-07-17 14:27:09
[post_content] =>
The Redesignation brings $10 million in funding to drive innovation, company formation, and economic growth.
The Center for Biotechnology (CFB) has announced its re-designated as a Center for Advanced Technology (CAT) by Empire State Development's Division of Science, Technology and Innovation (NYSTAR), a recognition that comes with $1 million in annual funding over the next ten years. The $10 million commitment underscores the CFB’s leadership in accelerating life science innovation, supporting early-stage technology development, and fueling economic growth through start-up formation and industry partnerships.
“The Center for Biotechnology has served as a critical bridge between academic research and commercial success,” said Dr. Clinton Rubin, Director of the Center for Biotechnology. “This re-designation ensures we can continue to expand our impact, helping innovators bring breakthrough technology to market and strengthening New York’s position as a leader in the bioscience industry.”
Empire State Development President, CEO and Commissioner Hope Knight said, “NYSTAR’s Centers for Advanced Technology are vital to our strategic efforts to grow New York’s economy and the state’s greater innovation ecosystem. By investing in the industries of tomorrow, New Yorkers benefit today through dynamic partnerships that help to create new jobs, generate more revenues, and encourage more companies to establish a footprint in communities all throughout the state.”
The Center for Biotechnology is located on the campus of Stony Brook University (SBU), the flagship research institution within the prestigious State University of New York (SUNY) system. Stony Brook University is recognized as a national and global leader in life sciences research, biomedical innovation, and clinical care. Situated on Long Island, New York, Stony Brook has built a formidable reputation as a hub for cutting-edge scientific discovery and translational medicine. The Center for Biotechnology builds upon these strengths by providing cutting-edge programming and competitive financial support to advance biomedical innovation and emerging company growth.
“We are excited to build upon the successful foundation of strong entrepreneurial networks, infrastructure, and programming that we have built over the last four decades” said Dr. Diane Fabel, Chief Operating Officer at the Center for Biotechnology. “The impacts we have had during our last designation period include over $1B in total economic impact with more than 1000 jobs created, and driving more than $315M in follow-on funding. We are excited to see those numbers continue to grow when we celebrate fifty years of hard work at the end of this redesignation period”.
As part of the New York State CAT program, the CFB will continue to work with emerging and established companies across the state to de-risk early-stage technologies, advance both technology and company value, foster public-private collaboration, and provide critical infrastructure for the region’s growing life science ecosystem. Additionally, the CFB team will continue its efforts to develop a life sciences workforce to support the region's bio-innovation economy with a specific emphasis on sectors deemed important to the NYS economy.
Dowload the full press release here.
[post_title] => Center for Biotechnology Announces Redesignation as New York State Center for Advanced Technology
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => redesignation2025
[to_ping] =>
[pinged] =>
[post_modified] => 2025-07-21 14:51:44
[post_modified_gmt] => 2025-07-21 18:51:44
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://centerforbiotechnology.org/?p=4906
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[1] => WP_Post Object
(
[ID] => 4488
[post_author] => 4
[post_date] => 2024-10-24 10:45:00
[post_date_gmt] => 2024-10-24 14:45:00
[post_content] =>
Governor Kathy Hochul has unveiled plans for New York BioGenesis Park, a groundbreaking $430 million Cell and Gene Therapy (CGT) Innovation Hub in Nassau County, Long Island. To be developed by The Albanese Organization, Inc., this state-of-the-art facility would catalyze CGT research, development, clinical manufacturing, and commercialization across New York State. With a historic $150 million state investment—the largest nationwide for a cell and gene therapy hub—NYBGP would accelerate the delivery of new therapies from lab to patient in New York's diverse communities. This transformative hub aims to establish New York as the leading global destination for CGT innovation, driving economic growth, attracting top talent, and revolutionizing patient care statewide and beyond.
The Center for Biotechnology is thrilled to be counted as a partner in this effort, and is looking forward to working with our colleagues in the initiative to help catalyze and accelerate life-changing therapies.
New York BioGenesis Park is envisioned as a cutting-edge, full-service campus dedicated to advancing cell and gene therapies and accelerating their commercialization. At full build-out, the 700,000-square-foot park would create an end-to-end Cell and Gene Therapy innovation and supply center, featuring interconnected areas for public engagement, research, manufacturing, and collaboration.
A cornerstone of New York BioGenesis Park is its incubator, supported by a $50 million investment from ESD's Long Island Investment Fund. This facility will empower early-stage therapeutic developers by offering state-of-the-art wet lab space, shared equipment, office space, and other essential resources. This nurturing environment will provide Cell and Gene Therapy companies with access to specialized equipment, mentoring, and stage-appropriate financial guidance. As a critical component of New York BioGenesis Park, the incubator is poised to catalyze the growth of promising Cell and Gene Therapy companies by providing them with resources and support, unlocking their potential for innovation and success.
New York BioGenesis Park would foster strong ties with academic and medical institutions throughout New York, creating a robust ecosystem for Cell and Gene Therapy innovation. Collaborating with the Empire State Cellular Therapy Consortium and world-class institutions like Cold Spring Harbor Laboratory, the Feinstein Institutes, Northwell Health, Roswell Park, Stony Brook University, Weill Cornell, Columbia University and others around the state, New York BioGenesis Park would enhance research synergies and accelerate medical breakthroughs. This ecosystem would bring together experts in advanced Cell and Gene Therapy therapies, offering specialized facilities, services, and resources to both tenants and collaborating institutions. By facilitating cutting-edge science, innovative technology development and novel approaches to clinical trials, New York BioGenesis Park would ensure New York's institutions remain globally competitive in groundbreaking Cell and Gene Therapy research and commercialization.
Read the press release of Governor Hochul's announcement here:
https://www.governor.ny.gov/news/governor-hochul-launches-next-phase-long-islands-nation-leading-cell-and-gene-therapy
[post_title] => Long Island to be location for Nation-Leading Cell and Gene Therapy Innovation Hub: New York BioGenesis Park.
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => long-island-to-be-location-for-nation-leading-cell-and-gene-therapy-innovation-hub-new-york-biogenesis-park
[to_ping] =>
[pinged] =>
[post_modified] => 2025-02-26 16:35:20
[post_modified_gmt] => 2025-02-26 21:35:20
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://centerforbiotechnology.org/?p=4488
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[2] => WP_Post Object
(
[ID] => 3811
[post_author] => 4
[post_date] => 2021-08-30 15:39:32
[post_date_gmt] => 2021-08-30 15:39:32
[post_content] =>
The BBCetc “From the Sources’ Mouth,” fall event is a free, virtual 3-day SBIR/STTR training with program managers from 11 federal agencies.
Each will present a 30-minute overview of their agency’s mission along with tips for creating a successful proposal. Everyone who attends will have time for Q&A as well as the opportunity to sign up for special one-on-one discussions with agency officers.
1 registration. 3 days. 11 SBIR/STTR agencies. One registration gives you access for all three days. Detailed agenda coming soon so you can choose which presenters you want to see.
Sept. 14, 2021
9:00 am - 5:00 pm (EST)
Granting Agencies
SBA, NIH, NSF, DOC-NOAA, DOE, USDA, NIAD
Sept. 15, 2021
9:00 am - 5:00 pm (EST)
Contracting Agencies
SBA, EPA, BARDA, NASA, DOT, DOEd, CDMRP
Sept. 16, 2021
9:00 am - 5:00 pm (EST)
DOD & Components
DOD, DHA, DARPA, SPARTN, NavSea, Air Force/AFWERX, DHS
Register Now
[post_title] => “From the Sources’ Mouth" SBIR/STTR training Program
[post_excerpt] =>
[post_status] => publish
[comment_status] => closed
[ping_status] => closed
[post_password] =>
[post_name] => from-the-sources-mouth-sbir-sttr-training-program
[to_ping] =>
[pinged] =>
[post_modified] => 2021-09-20 16:03:09
[post_modified_gmt] => 2021-09-20 16:03:09
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://centerforbiotechnology.org/?p=3811
[menu_order] => 39
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[3] => WP_Post Object
(
[ID] => 4431
[post_author] => 4
[post_date] => 2024-07-08 10:28:11
[post_date_gmt] => 2024-07-08 14:28:11
[post_content] =>
The Long Island Manufacturing Extension Partnership (LIMEP) has a new Manufacturing Grant Opportunity for modernization and equipment needs.
Empire State Development (ESD) has a new grant opportunity to expand manufacturing businesses. Applications are due July 31st by 4PM for the ESD Manufacturing Grant program. Assisting small Long Island manufacturing companies is a top priority of ESD and the regional MEP. Those interested in applying are strongly encouraged to get in touch with the team at LIMEP: https://www.stonybrook.edu/mtrc/
Additional information about the Small Manufacturer Modernization Grant Program can be found on the ESD website: https://regionalcouncils.ny.gov/long-island
[post_title] => Small Manufacturer Modernization Grant
[post_excerpt] => Empire State Development (ESD) has a new grant opportunity to expand manufacturing businesses. Applications are due July 31st by 4PM for the ESD Manufacturing Grant program.
[post_status] => publish
[comment_status] => closed
[ping_status] => closed
[post_password] =>
[post_name] => small-manufacturer-modernization-grant
[to_ping] =>
[pinged] =>
[post_modified] => 2024-11-19 10:49:48
[post_modified_gmt] => 2024-11-19 15:49:48
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://centerforbiotechnology.org/?p=4431
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[4] => WP_Post Object
(
[ID] => 3181
[post_author] => 3
[post_date] => 2019-02-28 16:35:02
[post_date_gmt] => 2019-02-28 16:35:02
[post_content] => Center for Biotechnology Director Dr. Clinton Rubin has co-authored a recent article published in Nature Reviews Endocrinology "“Combating osteoporosis and obesity with exercise: leveraging cell mechanosensitivity”. Abstract below, full article here.
Abstract:
Osteoporosis, a condition of skeletal decline that undermines quality of life, is treated with pharmacological interventions that are associated with poor adherence and adverse effects. Complicating efforts to improve clinical outcomes, the incidence of obesity is increasing, predisposing the population to a range of musculoskeletal complications and metabolic disorders. Pharmacological management of obesity has yet to deliver notable reductions in weight and debilitating complications are rarely avoided. By contrast, exercise shows promise as a non-invasive and non-pharmacological method of regulating both osteoporosis and obesity. The principal components of exercise — mechanical signals — promote bone and muscle anabolism while limiting formation and expansion of fat mass. Mechanical regulation of bone and marrow fat might be achieved by regulating functions of differentiated cells in the skeletal tissue while biasing lineage selection of their common progenitors — mesenchymal stem cells. An inverse relationship between adipocyte versus osteoblast fate selection from stem cells is implicated in clinical conditions such as childhood obesity and increased marrow adiposity in type 2 diabetes mellitus, as well as contributing to skeletal frailty. Understanding how exercise-induced mechanical signals can be used to improve bone quality while decreasing fat mass and metabolic dysfunction should lead to new strategies to treat chronic diseases such as osteoporosis and obesity.
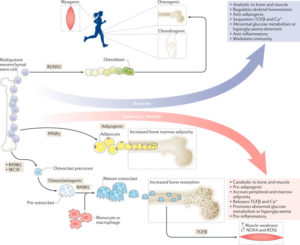 [post_title] => “Combating osteoporosis and obesity with exercise: leveraging cell mechanosensitivity”
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => combating-osteoporosis-and-obesity-with-exercise-leveraging-cell-mechanosensitivity
[to_ping] =>
[pinged] =>
[post_modified] => 2019-04-09 14:40:26
[post_modified_gmt] => 2019-04-09 14:40:26
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://centerforbiotechnology.org/?p=3181
[menu_order] => 100
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
)
[post_count] => 5
[current_post] => -1
[before_loop] => 1
[in_the_loop] =>
[post] => WP_Post Object
(
[ID] => 4906
[post_author] => 4
[post_date] => 2025-07-17 10:27:09
[post_date_gmt] => 2025-07-17 14:27:09
[post_content] =>
[post_title] => “Combating osteoporosis and obesity with exercise: leveraging cell mechanosensitivity”
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => combating-osteoporosis-and-obesity-with-exercise-leveraging-cell-mechanosensitivity
[to_ping] =>
[pinged] =>
[post_modified] => 2019-04-09 14:40:26
[post_modified_gmt] => 2019-04-09 14:40:26
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://centerforbiotechnology.org/?p=3181
[menu_order] => 100
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
)
[post_count] => 5
[current_post] => -1
[before_loop] => 1
[in_the_loop] =>
[post] => WP_Post Object
(
[ID] => 4906
[post_author] => 4
[post_date] => 2025-07-17 10:27:09
[post_date_gmt] => 2025-07-17 14:27:09
[post_content] =>
The Redesignation brings $10 million in funding to drive innovation, company formation, and economic growth.
The Center for Biotechnology (CFB) has announced its re-designated as a Center for Advanced Technology (CAT) by Empire State Development's Division of Science, Technology and Innovation (NYSTAR), a recognition that comes with $1 million in annual funding over the next ten years. The $10 million commitment underscores the CFB’s leadership in accelerating life science innovation, supporting early-stage technology development, and fueling economic growth through start-up formation and industry partnerships.
“The Center for Biotechnology has served as a critical bridge between academic research and commercial success,” said Dr. Clinton Rubin, Director of the Center for Biotechnology. “This re-designation ensures we can continue to expand our impact, helping innovators bring breakthrough technology to market and strengthening New York’s position as a leader in the bioscience industry.”
Empire State Development President, CEO and Commissioner Hope Knight said, “NYSTAR’s Centers for Advanced Technology are vital to our strategic efforts to grow New York’s economy and the state’s greater innovation ecosystem. By investing in the industries of tomorrow, New Yorkers benefit today through dynamic partnerships that help to create new jobs, generate more revenues, and encourage more companies to establish a footprint in communities all throughout the state.”
The Center for Biotechnology is located on the campus of Stony Brook University (SBU), the flagship research institution within the prestigious State University of New York (SUNY) system. Stony Brook University is recognized as a national and global leader in life sciences research, biomedical innovation, and clinical care. Situated on Long Island, New York, Stony Brook has built a formidable reputation as a hub for cutting-edge scientific discovery and translational medicine. The Center for Biotechnology builds upon these strengths by providing cutting-edge programming and competitive financial support to advance biomedical innovation and emerging company growth.
“We are excited to build upon the successful foundation of strong entrepreneurial networks, infrastructure, and programming that we have built over the last four decades” said Dr. Diane Fabel, Chief Operating Officer at the Center for Biotechnology. “The impacts we have had during our last designation period include over $1B in total economic impact with more than 1000 jobs created, and driving more than $315M in follow-on funding. We are excited to see those numbers continue to grow when we celebrate fifty years of hard work at the end of this redesignation period”.
As part of the New York State CAT program, the CFB will continue to work with emerging and established companies across the state to de-risk early-stage technologies, advance both technology and company value, foster public-private collaboration, and provide critical infrastructure for the region’s growing life science ecosystem. Additionally, the CFB team will continue its efforts to develop a life sciences workforce to support the region's bio-innovation economy with a specific emphasis on sectors deemed important to the NYS economy.
Dowload the full press release here.
[post_title] => Center for Biotechnology Announces Redesignation as New York State Center for Advanced Technology
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => redesignation2025
[to_ping] =>
[pinged] =>
[post_modified] => 2025-07-21 14:51:44
[post_modified_gmt] => 2025-07-21 18:51:44
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://centerforbiotechnology.org/?p=4906
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[comment_count] => 0
[current_comment] => -1
[found_posts] => 274
[max_num_pages] => 92
[max_num_comment_pages] => 0
[is_single] =>
[is_preview] =>
[is_page] =>
[is_archive] =>
[is_date] =>
[is_year] =>
[is_month] =>
[is_day] =>
[is_time] =>
[is_author] =>
[is_category] =>
[is_tag] =>
[is_tax] =>
[is_search] =>
[is_feed] =>
[is_comment_feed] =>
[is_trackback] =>
[is_home] => 1
[is_privacy_policy] =>
[is_404] =>
[is_embed] =>
[is_paged] =>
[is_admin] =>
[is_attachment] =>
[is_singular] =>
[is_robots] =>
[is_favicon] =>
[is_posts_page] =>
[is_post_type_archive] =>
[query_vars_hash:WP_Query:private] => 325c4f9f1aebaa5ec90666fa54175ba0
[query_vars_changed:WP_Query:private] =>
[thumbnails_cached] =>
[allow_query_attachment_by_filename:protected] =>
[stopwords:WP_Query:private] =>
[compat_fields:WP_Query:private] => Array
(
[0] => query_vars_hash
[1] => query_vars_changed
)
[compat_methods:WP_Query:private] => Array
(
[0] => init_query_flags
[1] => parse_tax_query
)
[query_cache_key:WP_Query:private] =>
)
