Center for Biotechnology Director Dr. Clinton Rubin has co-authored a recent article published in Nature Reviews Endocrinology ““Combating osteoporosis and obesity with exercise: leveraging cell mechanosensitivity”. Abstract below, full article here.
Abstract:
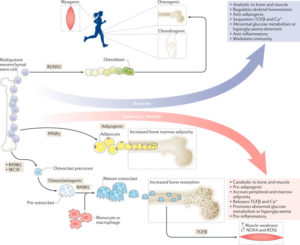
Osteoporosis, a condition of skeletal decline that undermines quality of life, is treated with pharmacological interventions that are associated with poor adherence and adverse effects. Complicating efforts to improve clinical outcomes, the incidence of obesity is increasing, predisposing the population to a range of musculoskeletal complications and metabolic disorders. Pharmacological management of obesity has yet to deliver notable reductions in weight and debilitating complications are rarely avoided. By contrast, exercise shows promise as a non-invasive and non-pharmacological method of regulating both osteoporosis and obesity. The principal components of exercise — mechanical signals — promote bone and muscle anabolism while limiting formation and expansion of fat mass. Mechanical regulation of bone and marrow fat might be achieved by regulating functions of differentiated cells in the skeletal tissue while biasing lineage selection of their common progenitors — mesenchymal stem cells. An inverse relationship between adipocyte versus osteoblast fate selection from stem cells is implicated in clinical conditions such as childhood obesity and increased marrow adiposity in type 2 diabetes mellitus, as well as contributing to skeletal frailty. Understanding how exercise-induced mechanical signals can be used to improve bone quality while decreasing fat mass and metabolic dysfunction should lead to new strategies to treat chronic diseases such as osteoporosis and obesity.